cl2 lewis structure
For ClO- 2Cl is the less electronegative atom. According to the lewis structure of Cl 2 O there are two lone pairs on.
 |
| Cl2 Lewis Structure Lewis Dot Structure For Cl2 Lewis Structure Of Cl2 Chlorine Lewis Structure Youtube |
Each dot represents a valence electron each line represents a bonded pair of electrons and each Cl represents a chlorine atom.
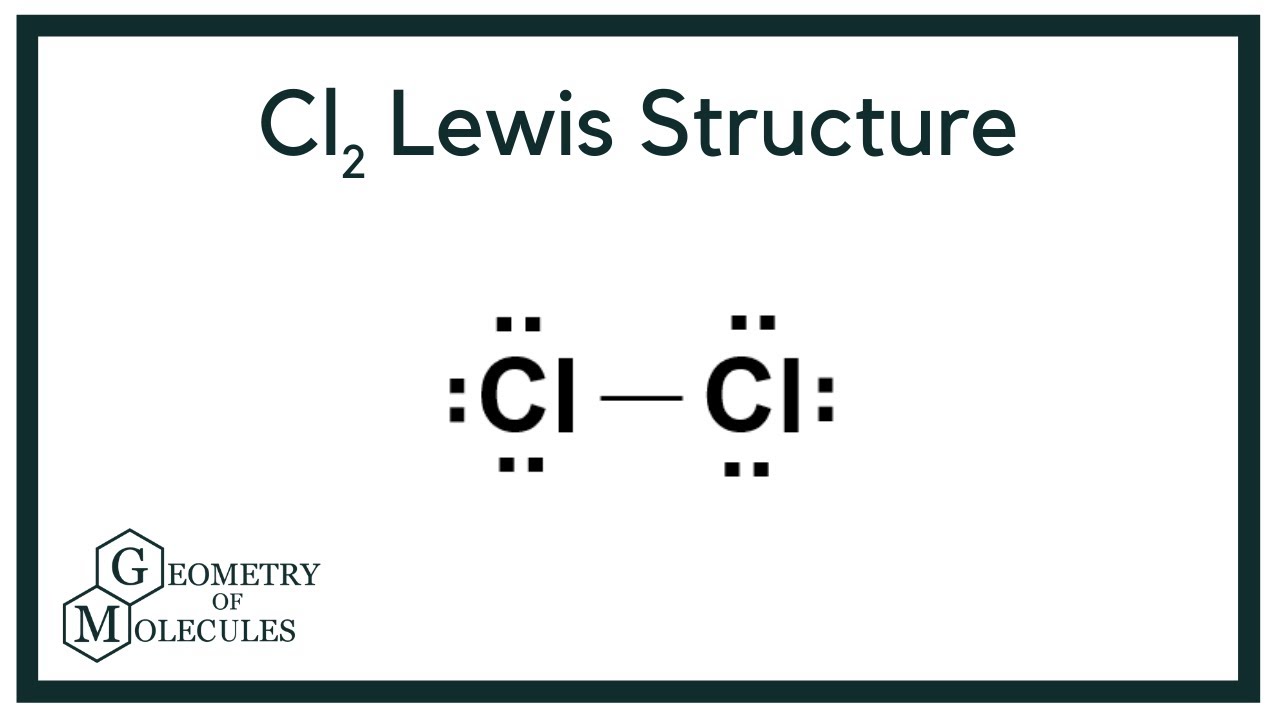
. Remember that the negative sign counts as one valence electron. A step-by-step explanation of how to draw the Cl2 Lewis Dot Structure Chlorine gasFor the Cl2 structure use the periodic table to find the total number of. The molecular shape of Cl2 is linear. If there are resonance forms write all reasonable resonance forms in the box.
It tells us how electrons are organized around specific atoms in a molecule. CCl 2 F 2 d. CH2Cl2 lewis structure lone pairs. Cl 2 r.
The molecule is bifunctional consisting of both an alkyl chloride and an alcohol functional group s. Both the chlorine atoms have three lone pairs in each and carbon or hydrogen atom does not have any lone pairs. In the lewis structure of Cl 2 there is a single bond between the two chlorine atoms and on each chlorine atom there are three lone pairs. The Cl2 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the Cl2 molecule.
The trial structure is. For the Cl 2 Lewis structure there are a total of 14 valence electrons available. The hybridization of each chlorine atom in Cl2 is Sp³. CH2Cl2 Lewis structure For understanding the properties and structure of any chemical compounds including organic ones its lewis structure is of the utmost importance.
The geometry of the Cl2 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the Cl2 geometrical shape in which the electrons have from one another. You have 20 valence electrons in your trial structure. The valence electrons you have available are. The formal charge of Chlorine in the Cl2 lewis dot structure is zero.
Chlorine Cl 2 Molecule Lewis Structure. There are total of 20 valence electrons for the ClO2- Lewis structure. The skeleton structure is O-Cl-O. Carbon is the least electonegative atom so it goes at the center of the C 2 H 2 Cl 2 Lewis structure.
What is the Lewis dot structure of Cl2. According to the lewis structure of Beryllium chloride Beryllium is a central atom and it has only two bond pairs. Cl 2 is a green poisonous gas. So weve used all 20 valence electrons for the Cl2O Lewis structure.
Molecule Lewis Structure. As the Beryllium atom forms two bond pairs with two chlorine atoms its general formula will be AX2. In the Lewis structure for ClO2- we put Chlorine Cl at the center of the structure since it is the least electronegative. CF 2 H 2 e.
The Cl 2 Lewis structure is similar to F 2 Br 2 and I 2 since F Br and I are all in Group 7 and have 7 valence electrons. Lewis structure of Dichlorine monoxide Cl 2 O contains two Cl-O bonds. A find total number of Valence Electrons. Lewis Structures Shapes and Polarity W 319 Everett Community College Student Support Services Program Draw Lewis structures name shapes and indicate polar or non-polar for the following molecules.
Any given atom follows the octet rule although there are some exceptions to this rule. Name and write the correct Lewis structure in the adjacent box. It is very easy to draw the Cl 2 lewis structure. Lewis structure is a theory that helps in understanding the structure of a given compound based on the octet rule.
Beryllium chloride has a hexagonal crystal structure. Lewis structure of chlorine molecule contains only one Cl-Cl bond and each chlorine atom has three lone pairs. Its shape can easily be predicted by the following table. Cl 2 chlorine has two chlorine atoms.
In the lewis structure of CH 2 Cl 2 carbon atom is the center atom as it has the highest valancy four with comparing to hydrogen and chlorine atoms. BeCl2 is prepared by direct reaction of elemental beryllium with chlorine at high temperatures. Oxygen atom is the center atom and both chlorine atoms are located around that center oxygen atom. In this tutorial we will discuss Beryllium chloride BeCl2 lewis structure molecular geometry electron geometry hybridization polar or nonpolar its bond angle etc.
Hence the trial structure has the correct number of electrons. Chlorine has 7 valence e- per Cl atom. COMPOUND LEWIS STRUCTURE 1 Dichlorine monoxide Put oxygen in the middle FORMULA Cl2O 2 The sulfite ion FORMULA SO3 2 3 Sulfur trioxide FORMULA SO3 4 Ammonia common name - memorize. This Chlorine has eight valence electrons its outer shell is full.
In the lewis structure of CH 2 Cl 2 there are four single bonds around the carbon atom with two hydrogen atoms and two chlorine atoms attached to it and on each chlorine atom there are three lone pairs. CH 2 Cl 2 dichloromethane has one carbon atom two hydrogen atoms and two chlorine atoms. BeCl2 Lewis Structure. Chlorine is a diatomic molecule and contains only two chlorine atoms.
We have 6 8 and 16 then back to the central Oxygen 18 and 20 total valence electrons. Cl2 Lewis Structure. The Lewis structure of any given molecule helps to know the arrangement of atoms in the molecule bond formations and the lone pairs. Number of bond pairs.
Be Cl 2 BeCl 2. CH 2 O f. 1 Cl 2 O 1 e 1 7 2 6 1 20. Cl2 Lewis Structure.
Lewis Structure is a simple depiction of valence shell electrons in a molecule. Cl2 is non-polar in nature because of no dipole moment present in it. There are no charges on chlorine and oxygen atoms in Cl 2 O and we will learn how to draw the lewis structure of Cl 2 O in this tutorial. N 2 O i.
The total valence electron available for drawing the Cl2 lewis structure is 14. H 2 O m. It is also known as electron dot structures because electrons are represented by dots in. There are two atoms of Cl per Cl2.
See the Big List of Lewis Structures. Chlorine gas exists in diatomic form as Cl 2. So thats the Lewis structure for Cl2O. So Cl is the central atom.
Cl 2 is a greenish yellow gas and is a strong oxidizing agent. And the Oxygen in the center also has an octet. The Lewis Dot Structure for Cl 2. All these characteristics help in determining other properties of the molecule.
The Lewis dot structure for Cl2 the chemical formula for chlorine gas is written with two Cl symbols each of which is surrounded by three pairs of dots connected by a single line. A step-by-step explanation of how to draw the Cl2O Lewis Structure Dichlorine MonoxideFor the Cl2O structure use the periodic table to find the total numb. Dichloromethane has one carbon two hydrpgen and two chlorine atoms.
 |
| Cl2 Lewis Structure Dichlorine Youtube |
 |
| Cl2 Lewis Structure Geometry Hybridization And Polarity Techiescientist |
 |
| Cl2 Lewis Structure Drawing Method Of Cl2 Lewis Structure Molecular Geometry Of Cl2 Polarity And Hybridisation In Cl2 Molecule With Faqs |
 |
| Cl2 Lewis Structure How To Draw The Dot Structure For Cl2 Chemical Bonding Success In Chemistry |
 |
| Cl2 Lewis Structure Molecular Shape Polar Or Non Polar Dot Diagram |
Posting Komentar untuk "cl2 lewis structure"